With a view to produce efficient focused therapies for most cancers, scientists must isolate the genetic and phenotypic traits of most cancers cells, each inside and throughout completely different tumors, as a result of these variations affect how tumors reply to remedy.
A part of this work requires a deep understanding of the RNA or protein molecules every most cancers cell expresses, the place it’s positioned within the tumor, and what it seems like below a microscope.
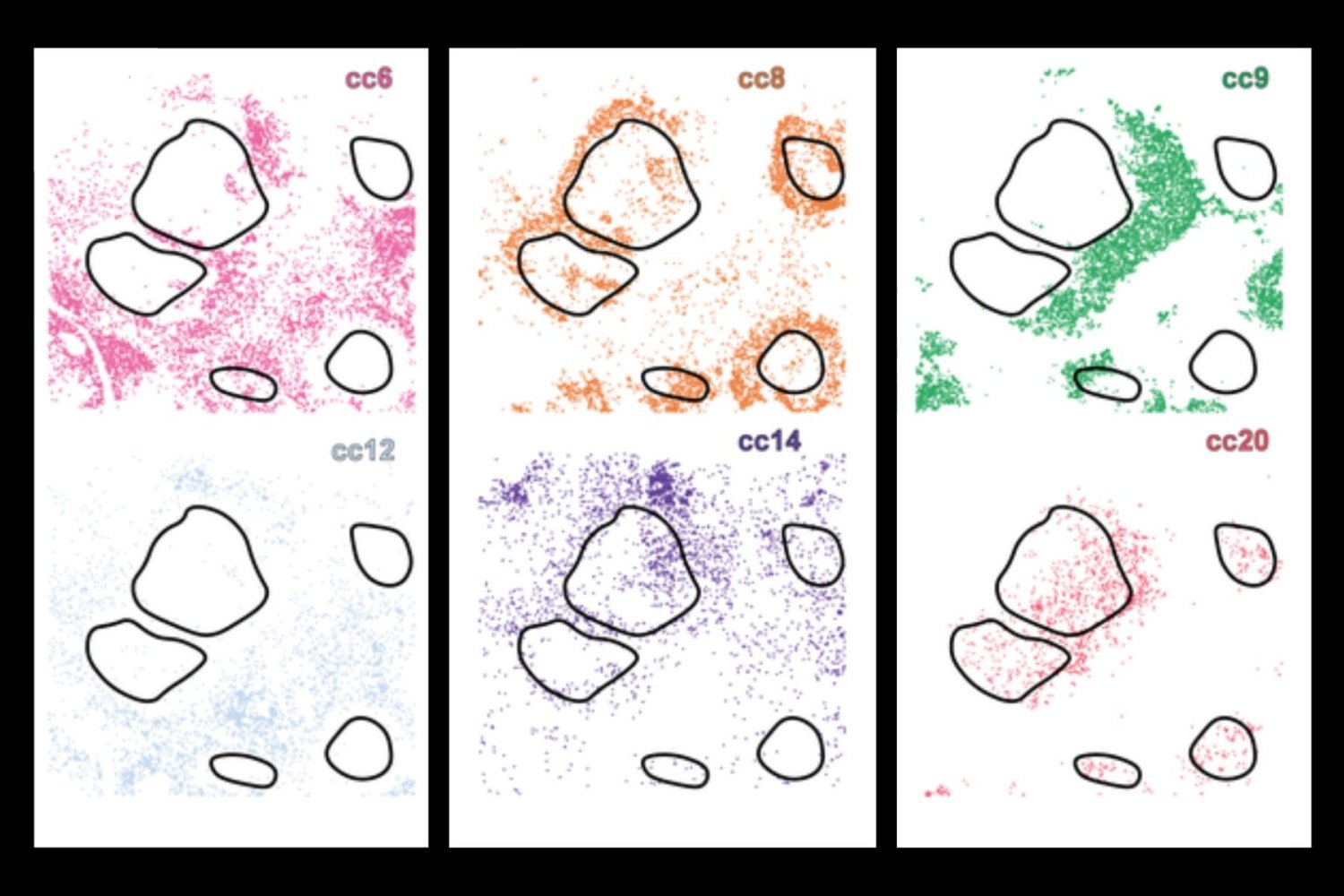
Historically, scientists have checked out a number of of those points individually, however now a brand new deep studying AI instrument, CellLENS (Cell Native Atmosphere and Neighborhood Scan), fuses all three domains collectively, utilizing a mixture of convolutional neural networks and graph neural networks to construct a complete digital profile for each single cell. This enables the system to group cells with comparable biology — successfully separating even people who seem very comparable in isolation, however behave otherwise relying on their environment.
The examine, printed just lately in Nature Immunology, particulars the outcomes of a collaboration between researchers from MIT, Harvard Medical Faculty, Yale College, Stanford College, and College of Pennsylvania — an effort led by Bokai Zhu, an MIT postdoc and member of the Broad Institute of MIT and Harvard and the Ragon Institute of MGH, MIT, and Harvard.
Zhu explains the affect of this new instrument: “Initially we might say, oh, I discovered a cell. That is known as a T cell. Utilizing the identical dataset, by making use of CellLENS, now I can say it is a T cell, and it’s presently attacking a particular tumor boundary in a affected person.
“I can use current data to raised outline what a cell is, what’s the subpopulation of that cell, what that cell is doing, and what’s the potential purposeful readout of that cell. This methodology could also be used to determine a brand new biomarker, which offers particular and detailed details about diseased cells, permitting for extra focused remedy growth.”
It is a important advance as a result of present methodologies typically miss important molecular or contextual data — for instance, immunotherapies might goal cells that solely exist on the boundary of a tumor, limiting efficacy. By utilizing deep studying, the researchers can detect many alternative layers of knowledge with CellLENS, together with morphology and the place the cell is spatially in a tissue.
When utilized to samples from wholesome tissue and a number of other sorts of most cancers, together with lymphoma and liver most cancers, CellLENS uncovered uncommon immune cell subtypes and revealed how their exercise and site relate to illness processes — resembling tumor infiltration or immune suppression.
These discoveries may assist scientists higher perceive how the immune system interacts with tumors and pave the way in which for extra exact most cancers diagnostics and immunotherapies.
“I’m extraordinarily excited by the potential of recent AI instruments, like CellLENS, to assist us extra holistically perceive aberrant mobile behaviors inside tissues,” says co-author Alex Ok. Shalek, the director of the Institute for Medical Engineering and Science (IMES), the J. W. Kieckhefer Professor in IMES and Chemistry, and an extramural member of the Koch Institute for Integrative Most cancers Analysis at MIT, in addition to an Institute member of the Broad Institute and a member of the Ragon Institute. “We are able to now measure an amazing quantity of details about particular person cells and their tissue contexts with cutting-edge, multi-omic assays. Successfully leveraging that information to appoint new therapeutic leads is a important step in creating improved interventions. When coupled with the proper enter information and cautious downsteam validations, such instruments promise to speed up our potential to positively affect human well being and wellness.”

With a view to produce efficient focused therapies for most cancers, scientists must isolate the genetic and phenotypic traits of most cancers cells, each inside and throughout completely different tumors, as a result of these variations affect how tumors reply to remedy.
A part of this work requires a deep understanding of the RNA or protein molecules every most cancers cell expresses, the place it’s positioned within the tumor, and what it seems like below a microscope.
Historically, scientists have checked out a number of of those points individually, however now a brand new deep studying AI instrument, CellLENS (Cell Native Atmosphere and Neighborhood Scan), fuses all three domains collectively, utilizing a mixture of convolutional neural networks and graph neural networks to construct a complete digital profile for each single cell. This enables the system to group cells with comparable biology — successfully separating even people who seem very comparable in isolation, however behave otherwise relying on their environment.
The examine, printed just lately in Nature Immunology, particulars the outcomes of a collaboration between researchers from MIT, Harvard Medical Faculty, Yale College, Stanford College, and College of Pennsylvania — an effort led by Bokai Zhu, an MIT postdoc and member of the Broad Institute of MIT and Harvard and the Ragon Institute of MGH, MIT, and Harvard.
Zhu explains the affect of this new instrument: “Initially we might say, oh, I discovered a cell. That is known as a T cell. Utilizing the identical dataset, by making use of CellLENS, now I can say it is a T cell, and it’s presently attacking a particular tumor boundary in a affected person.
“I can use current data to raised outline what a cell is, what’s the subpopulation of that cell, what that cell is doing, and what’s the potential purposeful readout of that cell. This methodology could also be used to determine a brand new biomarker, which offers particular and detailed details about diseased cells, permitting for extra focused remedy growth.”
It is a important advance as a result of present methodologies typically miss important molecular or contextual data — for instance, immunotherapies might goal cells that solely exist on the boundary of a tumor, limiting efficacy. By utilizing deep studying, the researchers can detect many alternative layers of knowledge with CellLENS, together with morphology and the place the cell is spatially in a tissue.
When utilized to samples from wholesome tissue and a number of other sorts of most cancers, together with lymphoma and liver most cancers, CellLENS uncovered uncommon immune cell subtypes and revealed how their exercise and site relate to illness processes — resembling tumor infiltration or immune suppression.
These discoveries may assist scientists higher perceive how the immune system interacts with tumors and pave the way in which for extra exact most cancers diagnostics and immunotherapies.
“I’m extraordinarily excited by the potential of recent AI instruments, like CellLENS, to assist us extra holistically perceive aberrant mobile behaviors inside tissues,” says co-author Alex Ok. Shalek, the director of the Institute for Medical Engineering and Science (IMES), the J. W. Kieckhefer Professor in IMES and Chemistry, and an extramural member of the Koch Institute for Integrative Most cancers Analysis at MIT, in addition to an Institute member of the Broad Institute and a member of the Ragon Institute. “We are able to now measure an amazing quantity of details about particular person cells and their tissue contexts with cutting-edge, multi-omic assays. Successfully leveraging that information to appoint new therapeutic leads is a important step in creating improved interventions. When coupled with the proper enter information and cautious downsteam validations, such instruments promise to speed up our potential to positively affect human well being and wellness.”



