An unlimited search of pure range has led scientists at MIT’s McGovern Institute for Mind Analysis and the Broad Institute of MIT and Harvard to uncover historic programs with potential to increase the genome modifying toolbox.
These programs, which the researchers name TIGR (Tandem Interspaced Information RNA) programs, use RNA to information them to particular websites on DNA. TIGR programs will be reprogrammed to focus on any DNA sequence of curiosity, they usually have distinct practical modules that may act on the focused DNA. Along with its modularity, TIGR may be very compact in comparison with different RNA-guided programs, like CRISPR, which is a significant benefit for delivering it in a therapeutic context.
These findings are reported on-line Feb. 27 within the journal Science.
“This can be a very versatile RNA-guided system with a variety of various functionalities,” says Feng Zhang, the James and Patricia Poitras Professor of Neuroscience at MIT, who led the analysis. The TIGR-associated (Tas) proteins that Zhang’s crew discovered share a attribute RNA-binding part that interacts with an RNA information that directs it to a particular website within the genome. Some reduce the DNA at that website, utilizing an adjoining DNA-cutting phase of the protein. That modularity may facilitate instrument improvement, permitting researchers to swap helpful new options into pure Tas proteins.
“Nature is fairly unimaginable,” says Zhang, who can also be an investigator on the McGovern Institute and the Howard Hughes Medical Institute, a core member of the Broad Institute, a professor of mind and cognitive sciences and organic engineering at MIT, and co-director of the Okay. Lisa Yang and Hock E. Tan Heart for Molecular Therapeutics at MIT. “It’s received an incredible quantity of range, and we have now been exploring that pure range to search out new organic mechanisms and harnessing them for various functions to control organic processes,” he says. Beforehand, Zhang’s crew tailored bacterial CRISPR programs into gene modifying instruments which have remodeled fashionable biology. His crew has additionally discovered a wide range of programmable proteins, each from CRISPR programs and past.
Of their new work, to search out novel programmable programs, the crew started by zeroing in a structural function of the CRISPR-Cas9 protein that binds to the enzyme’s RNA information. That could be a key function that has made Cas9 such a strong instrument: “Being RNA-guided makes it comparatively straightforward to reprogram, as a result of we all know how RNA binds to different DNA or different RNA,” Zhang explains. His crew searched tons of of tens of millions of organic proteins with recognized or predicted buildings, searching for any that shared an identical area. To search out extra distantly associated proteins, they used an iterative course of: from Cas9, they recognized a protein referred to as IS110, which had beforehand been proven by others to bind RNA. They then zeroed in on the structural options of IS110 that allow RNA binding and repeated their search.
At this level, the search had turned up so many distantly associated proteins that they crew turned to synthetic intelligence to make sense of the record. “If you end up doing iterative, deep mining, the ensuing hits will be so various that they’re troublesome to research utilizing commonplace phylogenetic strategies, which depend on conserved sequence,” explains Guilhem Faure, a computational biologist in Zhang’s lab. With a protein giant language mannequin, the crew was capable of cluster the proteins they’d discovered into teams in keeping with their probably evolutionary relationships. One group set aside from the remainder, and its members had been significantly intriguing as a result of they had been encoded by genes with recurrently spaced repetitive sequences harking back to a vital part of CRISPR programs. These had been the TIGR-Tas programs.
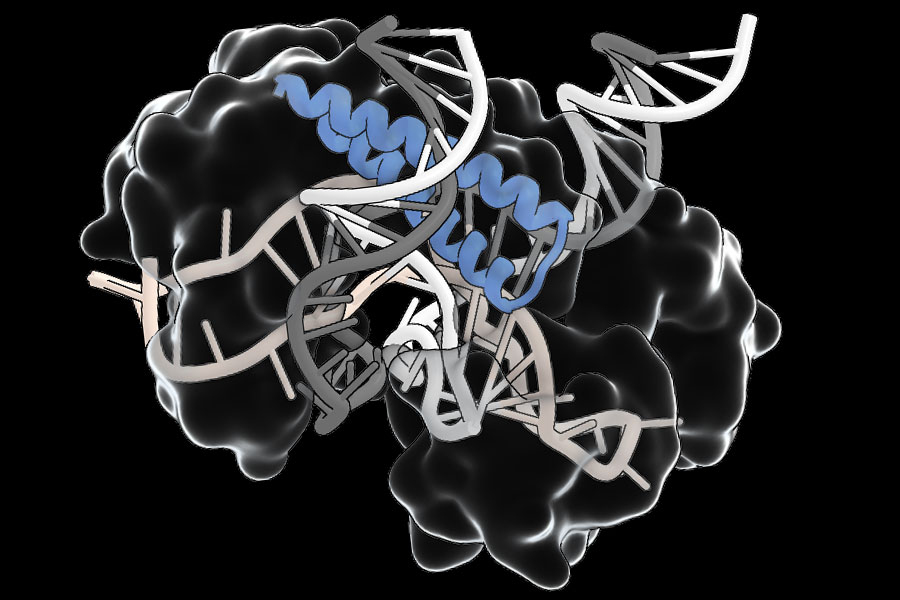
Zhang’s crew found greater than 20,000 completely different Tas proteins, largely occurring in bacteria-infecting viruses. Sequences inside every gene’s repetitive area — its TIGR arrays — encode an RNA information that interacts with the RNA-binding a part of the protein. In some, the RNA-binding area is adjoining to a DNA-cutting a part of the protein. Others seem to bind to different proteins, which suggests they could assist direct these proteins to DNA targets.
Zhang and his crew experimented with dozens of Tas proteins, demonstrating that some will be programmed to make focused cuts to DNA in human cells. As they give thought to creating TIGR-Tas programs into programmable instruments, the researchers are inspired by options that would make these instruments significantly versatile and exact.
They notice that CRISPR programs can solely be directed to segments of DNA which are flanked by brief motifs often called PAMs (protospacer adjoining motifs). TIGR Tas proteins, in distinction, don’t have any such requirement. “This implies theoretically, any website within the genome needs to be targetable,” says scientific advisor Rhiannon Macrae. The crew’s experiments additionally present that TIGR programs have what Faure calls a “dual-guide system,” interacting with each strands of the DNA double helix to house in on their goal sequences, which ought to guarantee they act solely the place they’re directed by their RNA information. What’s extra, Tas proteins are compact — 1 / 4 of the scale Cas9, on common — making them simpler to ship, which may overcome a significant impediment to therapeutic deployment of gene modifying instruments.
Excited by their discovery, Zhang’s crew is now investigating the pure function of TIGR programs in viruses, in addition to how they are often tailored for analysis or therapeutics. They’ve decided the molecular construction of one of many Tas proteins they discovered to work in human cells, and can use that data to information their efforts to make it extra environment friendly. Moreover, they notice connections between TIGR-Tas programs and sure RNA-processing proteins in human cells. “I feel there’s extra there to review by way of what a few of these relationships could also be, and it could assist us higher perceive how these programs are utilized in people,” Zhang says.
This work was supported by the Helen Hay Whitney Basis, Howard Hughes Medical Institute, Okay. Lisa Yang and Hock E. Tan Heart for Molecular Therapeutics, Broad Institute Programmable Therapeutics Reward Donors, Pershing Sq. Basis, William Ackman, Neri Oxman, the Phillips household, J. and P. Poitras, and the BT Charitable Basis.

An unlimited search of pure range has led scientists at MIT’s McGovern Institute for Mind Analysis and the Broad Institute of MIT and Harvard to uncover historic programs with potential to increase the genome modifying toolbox.
These programs, which the researchers name TIGR (Tandem Interspaced Information RNA) programs, use RNA to information them to particular websites on DNA. TIGR programs will be reprogrammed to focus on any DNA sequence of curiosity, they usually have distinct practical modules that may act on the focused DNA. Along with its modularity, TIGR may be very compact in comparison with different RNA-guided programs, like CRISPR, which is a significant benefit for delivering it in a therapeutic context.
These findings are reported on-line Feb. 27 within the journal Science.
“This can be a very versatile RNA-guided system with a variety of various functionalities,” says Feng Zhang, the James and Patricia Poitras Professor of Neuroscience at MIT, who led the analysis. The TIGR-associated (Tas) proteins that Zhang’s crew discovered share a attribute RNA-binding part that interacts with an RNA information that directs it to a particular website within the genome. Some reduce the DNA at that website, utilizing an adjoining DNA-cutting phase of the protein. That modularity may facilitate instrument improvement, permitting researchers to swap helpful new options into pure Tas proteins.
“Nature is fairly unimaginable,” says Zhang, who can also be an investigator on the McGovern Institute and the Howard Hughes Medical Institute, a core member of the Broad Institute, a professor of mind and cognitive sciences and organic engineering at MIT, and co-director of the Okay. Lisa Yang and Hock E. Tan Heart for Molecular Therapeutics at MIT. “It’s received an incredible quantity of range, and we have now been exploring that pure range to search out new organic mechanisms and harnessing them for various functions to control organic processes,” he says. Beforehand, Zhang’s crew tailored bacterial CRISPR programs into gene modifying instruments which have remodeled fashionable biology. His crew has additionally discovered a wide range of programmable proteins, each from CRISPR programs and past.
Of their new work, to search out novel programmable programs, the crew started by zeroing in a structural function of the CRISPR-Cas9 protein that binds to the enzyme’s RNA information. That could be a key function that has made Cas9 such a strong instrument: “Being RNA-guided makes it comparatively straightforward to reprogram, as a result of we all know how RNA binds to different DNA or different RNA,” Zhang explains. His crew searched tons of of tens of millions of organic proteins with recognized or predicted buildings, searching for any that shared an identical area. To search out extra distantly associated proteins, they used an iterative course of: from Cas9, they recognized a protein referred to as IS110, which had beforehand been proven by others to bind RNA. They then zeroed in on the structural options of IS110 that allow RNA binding and repeated their search.
At this level, the search had turned up so many distantly associated proteins that they crew turned to synthetic intelligence to make sense of the record. “If you end up doing iterative, deep mining, the ensuing hits will be so various that they’re troublesome to research utilizing commonplace phylogenetic strategies, which depend on conserved sequence,” explains Guilhem Faure, a computational biologist in Zhang’s lab. With a protein giant language mannequin, the crew was capable of cluster the proteins they’d discovered into teams in keeping with their probably evolutionary relationships. One group set aside from the remainder, and its members had been significantly intriguing as a result of they had been encoded by genes with recurrently spaced repetitive sequences harking back to a vital part of CRISPR programs. These had been the TIGR-Tas programs.
Zhang’s crew found greater than 20,000 completely different Tas proteins, largely occurring in bacteria-infecting viruses. Sequences inside every gene’s repetitive area — its TIGR arrays — encode an RNA information that interacts with the RNA-binding a part of the protein. In some, the RNA-binding area is adjoining to a DNA-cutting a part of the protein. Others seem to bind to different proteins, which suggests they could assist direct these proteins to DNA targets.
Zhang and his crew experimented with dozens of Tas proteins, demonstrating that some will be programmed to make focused cuts to DNA in human cells. As they give thought to creating TIGR-Tas programs into programmable instruments, the researchers are inspired by options that would make these instruments significantly versatile and exact.
They notice that CRISPR programs can solely be directed to segments of DNA which are flanked by brief motifs often called PAMs (protospacer adjoining motifs). TIGR Tas proteins, in distinction, don’t have any such requirement. “This implies theoretically, any website within the genome needs to be targetable,” says scientific advisor Rhiannon Macrae. The crew’s experiments additionally present that TIGR programs have what Faure calls a “dual-guide system,” interacting with each strands of the DNA double helix to house in on their goal sequences, which ought to guarantee they act solely the place they’re directed by their RNA information. What’s extra, Tas proteins are compact — 1 / 4 of the scale Cas9, on common — making them simpler to ship, which may overcome a significant impediment to therapeutic deployment of gene modifying instruments.
Excited by their discovery, Zhang’s crew is now investigating the pure function of TIGR programs in viruses, in addition to how they are often tailored for analysis or therapeutics. They’ve decided the molecular construction of one of many Tas proteins they discovered to work in human cells, and can use that data to information their efforts to make it extra environment friendly. Moreover, they notice connections between TIGR-Tas programs and sure RNA-processing proteins in human cells. “I feel there’s extra there to review by way of what a few of these relationships could also be, and it could assist us higher perceive how these programs are utilized in people,” Zhang says.
This work was supported by the Helen Hay Whitney Basis, Howard Hughes Medical Institute, Okay. Lisa Yang and Hock E. Tan Heart for Molecular Therapeutics, Broad Institute Programmable Therapeutics Reward Donors, Pershing Sq. Basis, William Ackman, Neri Oxman, the Phillips household, J. and P. Poitras, and the BT Charitable Basis.




